Sinovac Ema
Subscribe to our daily news digest. It may interest you.
Ema Pruft Chinesischen Corona Impfstoff Von Sinovac Medqn
Chinas Sinovac Biotech will trial its COVID-19 vaccine in children and adolescents in South Africa as part of a global Phase III study Sinovac and local partner Numolux Group said on Thursday.

Sinovac ema. The following four vaccines are under review. Quindici giorni dopo il valore anticorpale in risposta al Covid-19 aumenta di 20 volte. Sputnik V Sinovac and Sinopharm which are still under review.
Covishield Sinopharm Sinovac Vaccines Are Most Widely Accepted by EU Countries After Those Authorised by EMA. Commenting on EMAs fourth ongoing rolling review of a shot developed by US. For now the EMA officially accepts Pfizer BioNTech Moderna.
Currently all countries recognize proof of vaccination with one of the vaccines approved by the European Medicines Agency EMA which are PfizerBioNTech Moderna AstraZeneca and Johnson and Johnson. AstraZeneca is still not sure if it needs a. The EMAs Committee for Medicinal Products for Human Use CHMP conducts a rolling review of the clinical trial data of the vaccines.
Sinovac Life Sciences Cos CoronaVac. Boom di anticorpi dopo la combinazione di due dosi di vaccino SinoVac più una terza dose di Pfizer. However the case is not the same with other vaccines such as Sinovac.
COVID-19 EU Travel. EUSchengen September 24 2021. Firm Novavax he said its manufacturing would be discussed further over the next few weeks and an overall conclusion of the review is possible before year-end.
Only after it decides that enough evidence is available can the developer apply for marketing authorization. He added EMA would continue to review data on German biotech firm CureVacs shot over the next few weeks before a conclusion can be drawn. 70 of Europeans Are Planning to Travel in Next 4 Months Research Reveals.
Европейскому агентству лекарственных средств ema не хватает данных по российской вакцине Спутник v.
Paul Ehrlich Institut News Start Of The Rolling Review Procedure For Sinovac S Whole Virus Vaccine Called Covid 19 Vero Cell Inactivated At The European Medicines Agency

Sinovac Asisi Ema Da Incelemede Guncel Yayin Tarihi 06 05 2021 Koln Radyosu Programm Cosmo Radio Wdr
So Will Die Ema Den Chinesischen Sinovac Impfstoff Prufen Zdfheute

Eu Travel Covishield Sinopharm Sinovac Vaccines Are Most Widely Accepted By Eu Countries After Those Authorised By Ema Schengenvisainfo Com

Eu Behorde Pruft Chinesischen Corona Impfstoff Von Sinovac Aktuell Europa Dw 04 05 2021
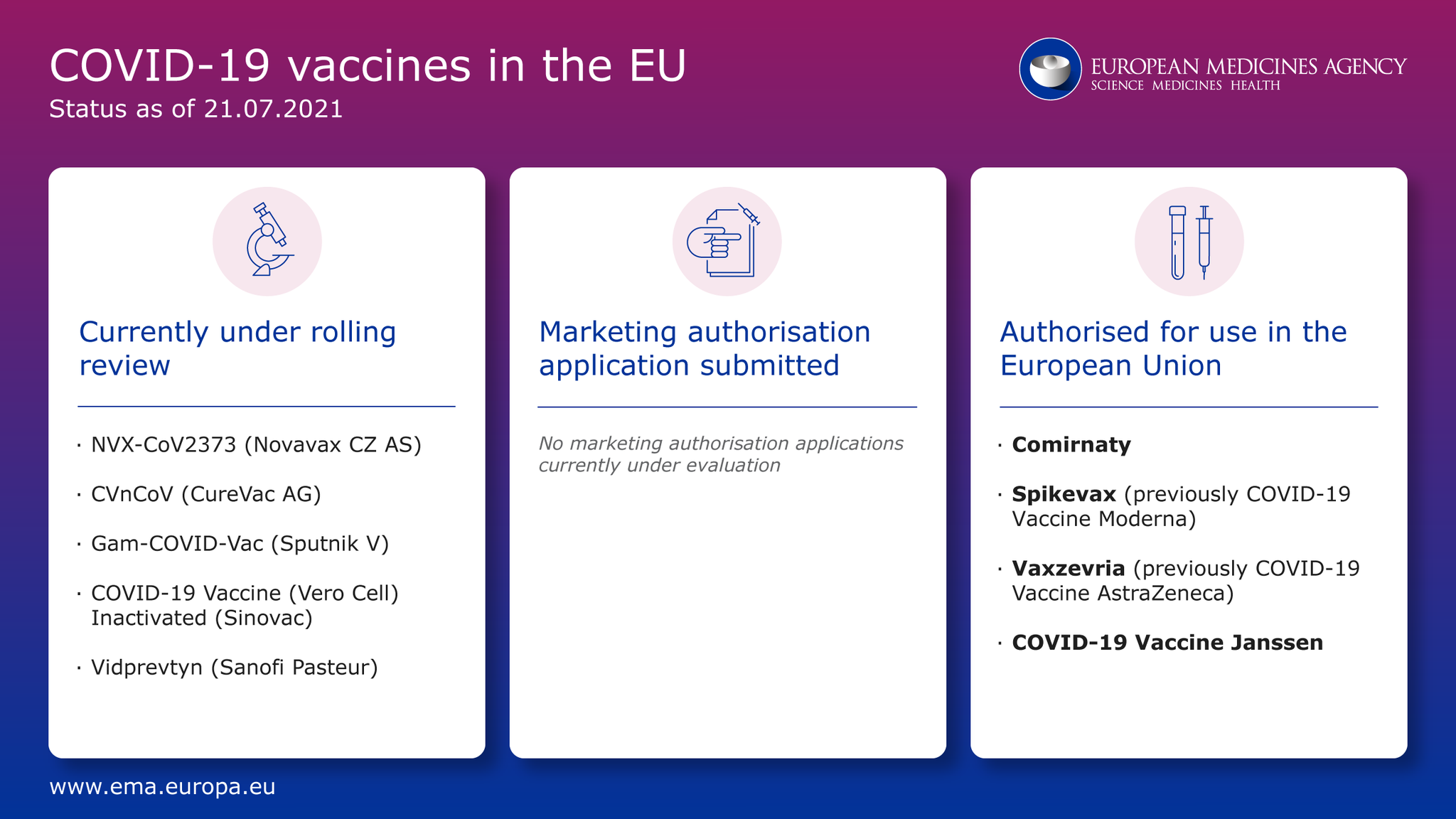
Eu Medicines Agency On Twitter Covid19vaccines Where Are We Now Ema Starts A New Rollingreview For Vidprevtyn Sanofi Pasteur No Marketing Authorisation Application Currently Under Review Https T Co T5aeifknsk
Eu Drug Regulator Starts Rolling Review Of Sinovac S Covid 19 Vaccine Cgtn
Ema Startet Prufung Des Chinesischen Impfstoffs Von Sinovac

Eu Regulator Begins Reviewing China S Sinovac Covid 19 Vaccine Coronavirus Pandemic News Al Jazeera
Chinesischer Corona Impfstoff Ema Startet Prufverfahren Von Sinovac Tagesschau De
Eu Regulator Starts Rolling Review Of China S Sinovac Covid 19 Vaccine Marketwatch

Corona Impfstoff Aus China Im Prufverfahren Der Ema Gelbe Liste

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters

Eu Regulator Examines Sinovac S Covid 19 Vaccine

Zulassung Oder Studie Welche Corona Impfstoffe Kommen Noch Das Erste

Covid 19 Vaccines Serious Adverse Events Explained

Vakzinbeschaffung Ema Startet Prufverfahren Fur Chinesischen Impfstoff Wiener Zeitung Online

Sinovac Ema Pruft Chinesischen Covid 19 Impfstoff Pz Pharmazeutische Zeitung

Ema Beginnt Mit Der Prufung Des Sinovac Impfstoffs Zm Online
